DOWNSTREAM INNOVATION
Leads To A New Level
In the pharmaceutical industry, there is a growing need for continuous and efficient processes to improve productivity and quality and to meet fluctuating demand.
In conventional biopharmaceutical manufacturing, equipment is designed for each process, and each requires capital investment.
In addition, each piece of equipment requires its own consumables, and the man-hours required for preparation, cleaning, and other tasks contribute to the high costs.
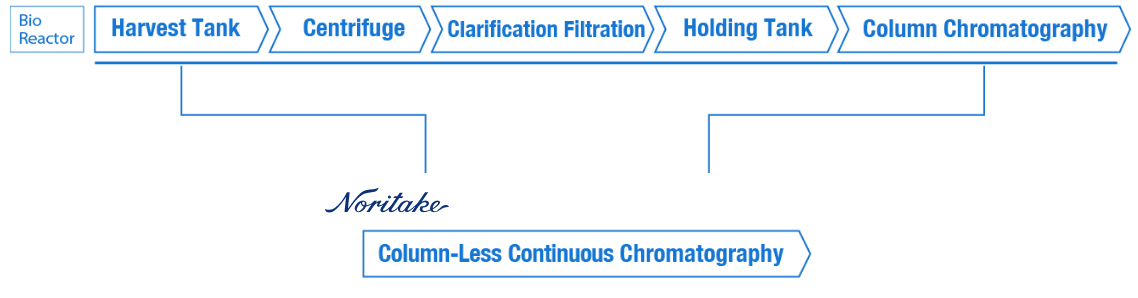
Column chromatography, which is commonly used in the purification process, is particularly problematic because batch processing is unavoidable and consumes large amounts of resins and buffers.
Noritake has realized a new technology that enables purification directly from the culture medium without column chromatography by using our fluid control technology.
The new downstream process provides an innovative way to improve productivity and reduce costs for continuous biopharmaceutical production.
Column Chromatography

Column-Less Technology
Noritake’s column-less technology continuously circulates resins, which were previously packed in columns, through a path combining a static mixer and a cyclone separator. This allows for continuous antibody adsorption and separation.
This eliminates the need for columns and enables continuous antibody purification by recovering and regenerating the resins. Additionally, by integrating the clarification and initial purification processes into one, it significantly reduces implementation costs, consumable costs, and operating time.
Intensified process and
productive platform

SAVE YOUR cost,
time
and space
Streamlined Performance,
Dependable Outcomes
Initial Cost
-50%
Consumables*
-80%
Footprint
-70%
Processing time
5 Days 1 Day
*Theoretical values based on a culture scale of
500L and an antibody expression level of 3g/L.
*Operating time includes preparation time
*Consumables refer to resin usage.
CONTINUOUS
PURIFICATION
PROCESS

CLEANING
It efficiently purges impurities from the resin.
ELUTION
Antibodies are isolated and collected from the resin in this step.
RESIN REGENERATION
After completing the series of purification processes, the resins are sent directly to the regeneration and re-equilibration process.
CORE TECHNOLOGY
Static Mixer
- Inline continuous mixing
- No power or drive components needed
- No significant shear forces applied
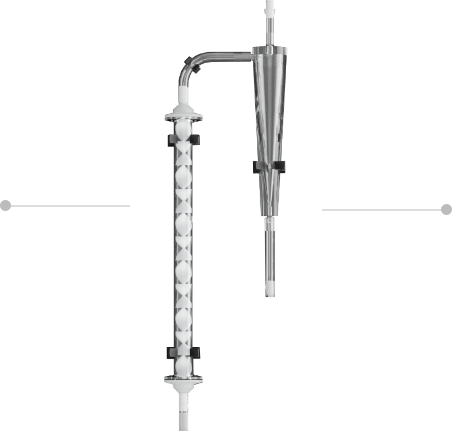
Cyclone Separator
- Inline continuous solid-liquid separation
- No power or drive components needed
- Specifically designed for Column-less Chromatography
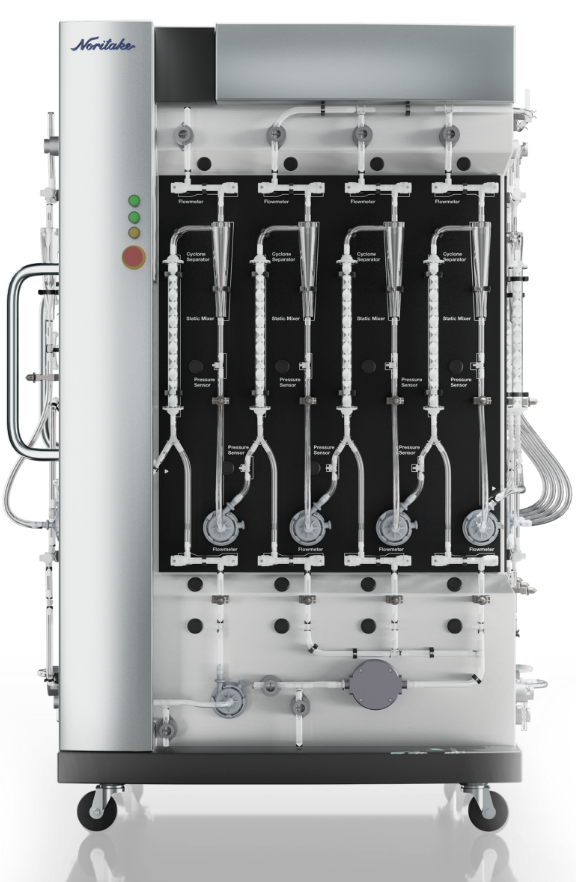
EQUIPMENT
SPECIFICATIONS
EQUIPMENT SIZE
( H x W x D )
1931 x 1400 x 1267 mm
( 76.02 x 55.12 x 49.89 inches )
PROCESS SPECIFICATIONS
Applicable Bioreactor Volume: 50 ~ 500 L
System Flow Rates: 2,000 mL/min
Total Processing Times: 50 min/50 L ~ 500 min/500 L *1
*1 Average estimate time of antibody purification
SINGLE-USE KIT
The liquid contact parts use single-use components, reducing the need for cleaning operations.
RESINS
We developed dedicated Protein A resin that enables capturing in a short time. It is different from the conventional resins for chromatography columns, and it realizes a completely new affinity chromatography.



